- Global | English
- Search
Universal Cell Platform(TyUCell?)refers to the use of gene editing technology to transform allogeneic immune cells to eliminate immune rejection.
lEARN MORE TyUCell?The Quikin CAR-T Platform(Quikin CART) generates T cell products with gene knockout and stable integration of CAR cassette in one step through CRISPR gene editing technology.
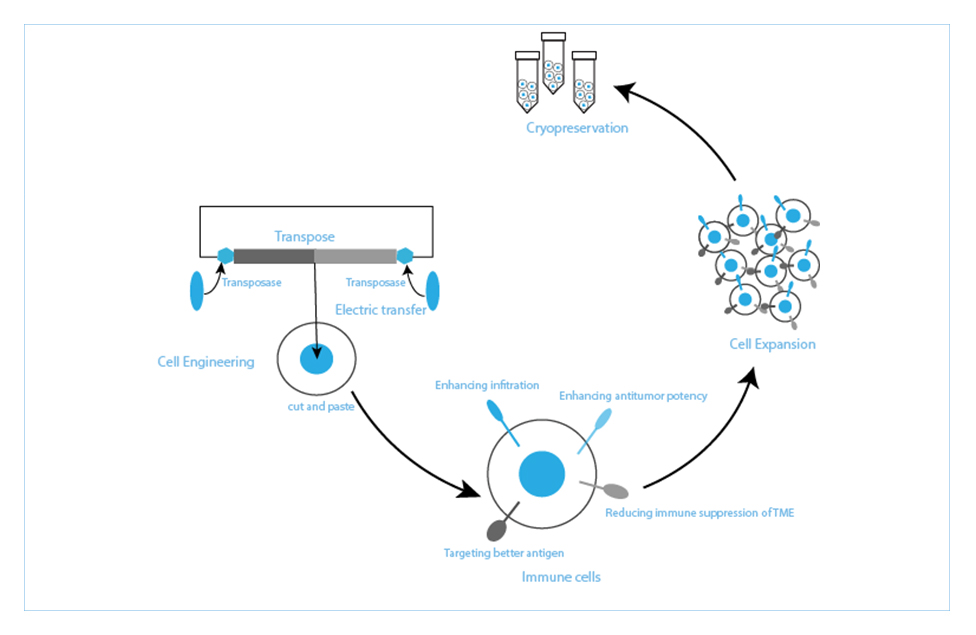
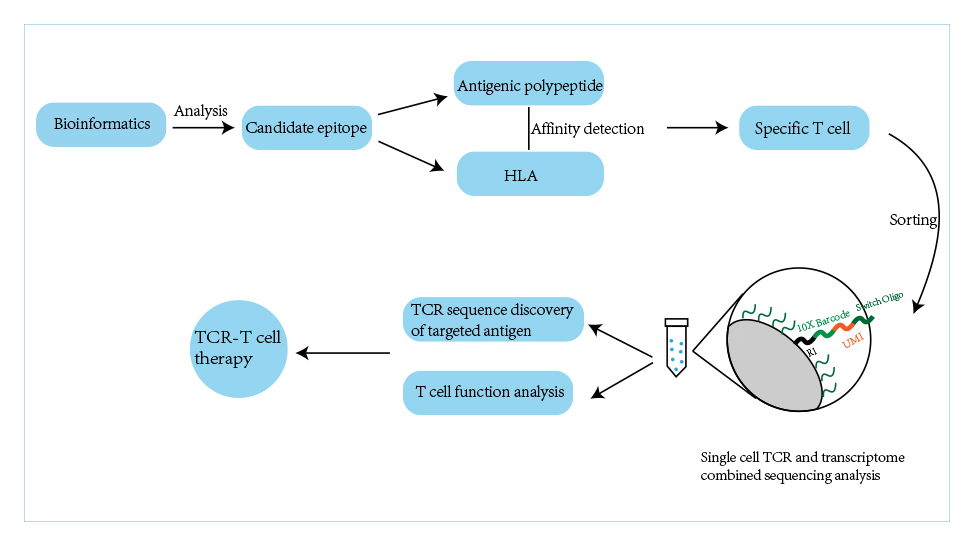
lEARN MORE Quikin CARTEnhanced T cell Platforms(HyperTCell?), including HyperCART ? and HyperTCRT , are mainly to solve the global highlighted problem in solid tumor therapy through genetic modification of T cells.
lEARN MORE HyperTCell?Enhanced T Cell Platforms (HyperTCell ®), including HyperCART® and HyperTCRT, are mainly to solve the global highlighted problem in solid tumor therapy through genetic modification of T cells. HyperCART® takes improving the clinical efficacy of CAR-T in solid tumors as its core purpose. Based on the concept of synthetic biology, HyperCART® adopts efficient gene delivery system to target tumor cells specifically, reverse the immuno suppressive state and improve the predicament of difficult infiltration and poor efficacy of traditional CAR-T in solid tumor therapy. HyperTCRT is based on the discovery and validation of TCR targeting tumor-specific neoantigen mutations, combined with efficient gene editing technology, to develop a new generation of enhanced TCR-T cells to improve the effectiveness of solid tumor therapy.